The definition of an acid or a base depends on the system you are using. In the Arrhenius definition of acids and bases, an acid is a substance that releases hydrogen ions when dissolved in water, and a base is a substance that releases hydroxide ions. Although in chemistry today, the Brønsted-Lowry definition has largely superseded it, under the Arrhenius definition it is possible to predict the result of the neutralization of an acid with a base, which is a salt and water.
If a chemist doesn't specify which acid-base system they are using, it can be assumed they are working within the Brønsted-Lowry system. A Brønsted acid is any substance that can donate a hydrogen ion. A Brønsted base is defined as any substance that can accept a hydrogen ion. In other words, a Brønsted acid is a proton donor, and a Brønsted base is a proton receiver. The Brønsted-Lowry system implies the acid-base reaction to occur within a system of conjugate acid-base pairs governed by an equilibrium. Acids increase the concentration of hydrogen ions, while bases decrease the concentration of hydrogen ions. The acidity or basicity of a solution can therefore can be measured by its hydrogen ion concentration (or pH, the negative logarithm of concentration).
The Lewis definition of acids (as electron pair receivers) and bases (as electron pair donors) is actually broader than either the Arrhenius or Brønsted-Lowry. The Lewis system provides a productive framework for viewing many chemical events from nucleophilic approach to the formation of coordination complexes, although the Brønsted-Lowry system is the traditional framework for discussion and problem solving involving acid-base equilibria.
As with Solutions, the previous chapter, every MCAT will have a number of questions deriving from the material in Acids & Bases. Although big, multi-variable quantitative acid-base equilibrium questions are a standard of Chem 101, you are not likely to run into such questions on the MCAT. However, the MCAT definitely will call on you to demonstrate that you understand the basic concepts of acid-base equilibrium. Buffers & indicators are MCAT favorites, as well as titration curves.
WikiPremed Resources
Acids & Bases Practice Items
Conceptual Vocabulary Self-Test
Basic Terms Crossword Puzzle
Basic Puzzle Solution
Conceptual Vocabulary for Acids & Bases
Acids & Bases
An acid is traditionally considered any chemical compound that, when dissolved in water, gives a solution with a pH less than 7.0.
A base is most commonly thought of as a substance that can accept protons.
pH is a measure of the acidity or alkalinity of a solution.
A strong acid is an acid that dissociates completely in an aqueous solution.
Acetic acid, also known as ethanoic acid, is an organic chemical compound best recognized for giving vinegar its sour taste and pungent smell.
Carboxylic acids are organic acids characterized by the presence of a carboxyl group.
Sodium hydroxide, also known as lye, caustic soda and sodium hydrate, is a caustic metallic base.
Hydronium is the common name for the cation derived from protonation of water. It is the simplest type of an oxonium ion.
An acid-base reaction is a chemical reaction that occurs between a proton donor and a proton receiver.
A Lewis base is any molecule or ion that can form a new coordinate covalent bond, by donating a pair of electrons.
Amines and nitrogen-containing heterocyclic compounds are organic bases.
Amines are organic compounds and a type of functional group that contain nitrogen as the key atom. Structurally amines resemble ammonia, wherein one or more hydrogen atoms are replaced by alkyl and aryl groups.
Hydrochloric acid is the aqueous solution of hydrogen chloride gas.
A Lewis acid can accept a pair of electrons and form a coordinate covalent bond.
A weak base is a chemical base that does not ionize fully in an aqueous solution
A weak acid is an acid that does not ionize in solution to a significant extent.
An alkoxide is the conjugate base of an alcohol and therefore consists of an organic group bonded to a negatively charged oxygen atom.
An alkali is a basic, ionic salt of a group I or group II element.
Sodium carbonate is a sodium salt of carbonic acid.
An acid dissociation constant is an equilibrium constant for the dissociation of a weak acid.
Buffer solutions are solutions that resist change in Hydronium ion and the hydroxide ion concentration (and consequently pH) upon addition of small amounts of acid or base, or upon dilution.
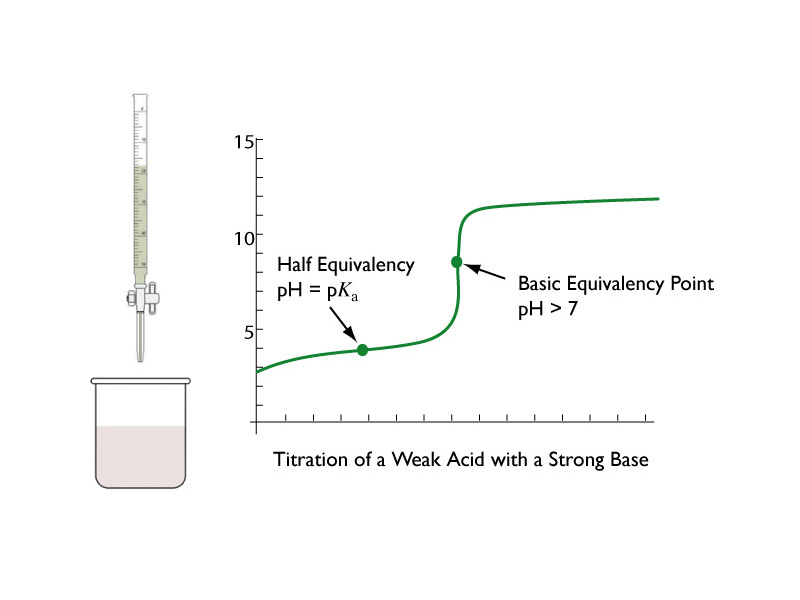
An acid-base titration is a volumetric method in chemistry that allows quantitative analysis of the concentration of an unknown acid or base solution, making use of the neutralization reaction that occurs between acids and bases.
Protonation is the addition of a hydrogen ion to an atom, molecule, or ion.
A pH indicator is a halochromic chemical compound that is added in small amounts to a solution so that the pH of the solution can be determined easily.
An acetate, or ethanoate, is a salt or ester of acetic acid.
Ammonia is a compound consisting of one nitrogen atom singly bound to three hydrogen atoms, normally encountered as a gas with a characteristic pungent odor.
Hydrogen chloride has the formula HCl.
Sulfuric acid is a strong mineral acid once known as oil of vitriol. Each molecule contains a sulfur atom, two hydrogen atoms and four oxygen atoms.
The equivalence point or stoichiometric point occurs during a chemical titration when the amount of titrant added is equivalent, or equal, to the amount of analyte present in the sample.
A mineral acid is an acid derived from inorganic substances by chemical reaction as opposed to organic acids.
Neutralization is a chemical reaction, also called a water forming reaction, in which an acid and a base react and produce a salt and water.
Phenol, also known under an older name of carbolic acid, possesses a structure consisting of a hydroxyl group bonded to a phenyl ring.
Benzoic acid is a colorless crystalline solid and the simplest aromatic carboxylic acid.
An oxoacid is an acid which contains oxygen.
Nitric acid, also known as aqua fortis and spirit of nitre, is an aqueous solution of hydrogen nitrate.
Phosphoric acid, also known as orthophosphoric acid, is a mineral acid having a molecular structure with one phosphorus atom, four oxygen atoms, and three hydrogen atoms.
A bicarbonate is an intermediate form in the deprotonation of carbonic acid.
Nitrogenous bases are organic compounds that owe their basic properties to the lone pair of electrons of a nitrogen atom.
Enols are alkenes with a hydroxyl group affixed to one of the carbon atoms composing the double bond.
A superbase is an extremely strong base.
A superacid is an acid with an acidity greater than that of 100% sulfuric acid.
The proton affinity of an anion or of a neutral atom or molecule is a measure of its gas-phase basicity.
Hydron is the general name for the positive hydrogen cation, used to reflect ions formed from the naturally abundant hydrogen, as opposed to proton, which only refers to the most common isotope of hydrogen.
A burette is a vertical cylindrical piece of laboratory glassware with a volumetric graduation on its full length and a precision tap, or stopcock, on the bottom.
Formic acid (systematically called methanoic acid) is the simplest carboxylic acid
Malonic acid is a dicarboxylic acid with two carboxyl groups bound to a central carbon.
Basic aromatic rings are aromatic rings in which the lone pair of electrons of a ring-nitrogen atom is not part of the aromatic system and extends in the plane of the ring.
Methylamine is a derivative of ammonia, wherein one H atom is replaced by a methyl group
A harpoon base is an organic base that is a very strong base but at the same time a poor nucleophile.