Interdisciplinary Note (2 of 22)
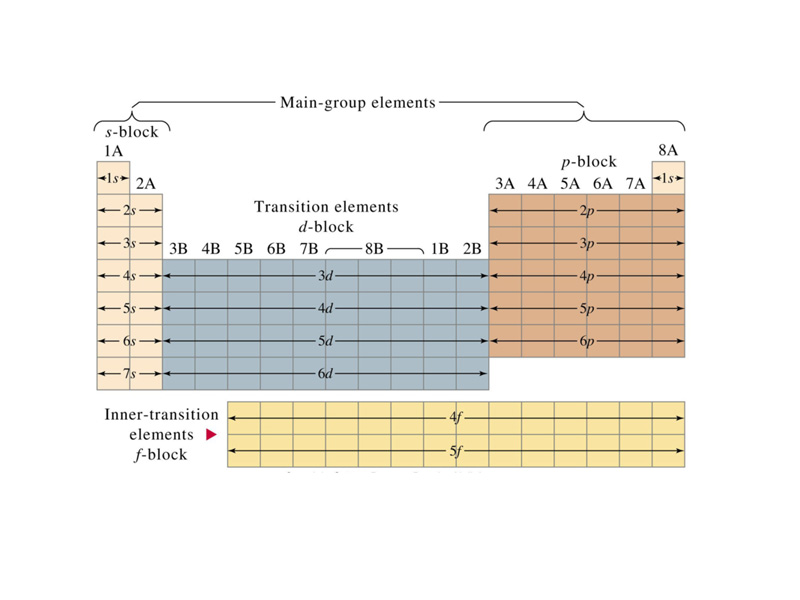
The periodic table represents how chemical properties repeat themselves from lightest to heaviest along the sequence of known elements. For example, an inert gas is always followed next in line by a highly reactive metal. Although Mendeleev's work preceded the development of atomic theory by thirty years, because the outer shell electron configuration plays a major roll in determining the chemical properties of an atom, the organization of the periodic table corresponds to the organization of atomic orbitals. Although the periodic table was introduced prior to the advent of quantum mechanics, one of the most important early insights to gain in learning chemistry is how the organization of the periodic table corresponds to electron configuration.
The design of the periodic table reflects the observation that many properties of the chemical elements are periodic functions of their atomic number. Along with an atom's valence shell configuration, the periodic properties of an element provide a thumbnail picture of its chemical behavior. The valence plus the atomic radius, ionization energy, electron affinity and electronegativity constitute the 'personality' of an element.
Only rarely to questions appear on the MCAT that ask you directly about periodic properties, but like so many of these early physical science chapters, the material is fundamental to much else which comes later. Of the periodic properties, the electronegativity is especially crucial. Electronegativity difference between bonded atoms determines bond polarity, for example. Understanding bond polarity is necessary for predicting intermolecular forces, solubility relationships or assigning oxidation numbers.